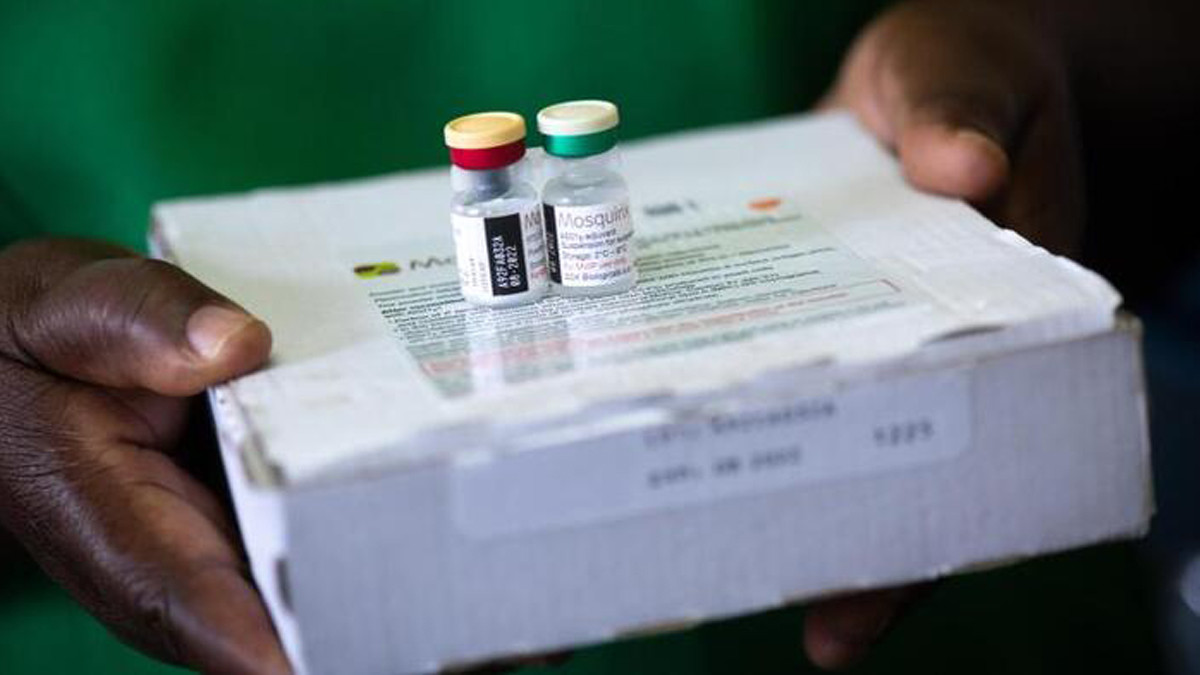
The United Nations Children's Fund (UNICEF) has awarded a contract for the first ever supply of a malaria vaccine to pharmaceutical company GSK with a value of up to USD 170 million.
The UN agency said in a statement that the landmark award will lead to 18 million doses of RTS, S being available over the next three years, potentially saving thousands of lives every year.
"This vaccine rollout gives a clear message to malaria vaccine developers to continue their work because malaria vaccines are needed and wanted," said Etleva Kadilli, Director of UNICEF's Supply Division. "We hope this is just the beginning. Continued innovation is needed to develop new and next-generation vaccines to increase available supply, and enable a healthier vaccine market. This is a giant step forward in our collective efforts to save children's lives and reduce the burden of malaria as part of wider malaria prevention and control programs."
According to WHO data, more than 30 countries have areas with moderate to high malaria transmission, where the vaccine could provide added protection against malaria to over 25 million children each year once the supply scales up.
The RTS, S malaria vaccine is the result of 35 years of research and development and is the first-ever vaccine against a parasitic disease. The vaccine acts against Plasmodium falciparum, the most deadly malaria parasite globally and the most prevalent in Africa.
In 2019, pilot routine vaccine use was launched in three countries - Ghana, Kenya, and Malawi - as part of the Malaria Vaccine Implementation Programme coordinated by WHO. The experience and evidence generated by the pilots informed WHO's recommendation in October 2021 for the widespread use of the first malaria vaccine in countries with moderate to high P. falciparum malaria transmission. Soon after, in December 2021, Gavi, the Vaccine Alliance's decision to provide funding for malaria vaccine programs in eligible countries opened the pathway for a broader roll-out of the vaccine.
"Lives are at stake, every day. WHO welcomes the progress to secure supply and timely access to vaccines so that more countries can begin to introduce this additional malaria prevention tool as rapidly as possible," said Dr. Kate O'Brien, WHO Director of the Department of Immunization, Vaccines, and Biologicals. "Given the initial limited supply, it is crucial that children living in areas where the risk of disease and need is highest are prioritized first."
This award is the culmination of 18 months of intensive preparation and consultation with industry and partners, UNICEF said.
Demand for the malaria vaccine is expected to be high among affected countries. As with any new vaccine, supply will be limited at first and will increase over time as manufacturing capacity ramps up to the level required.